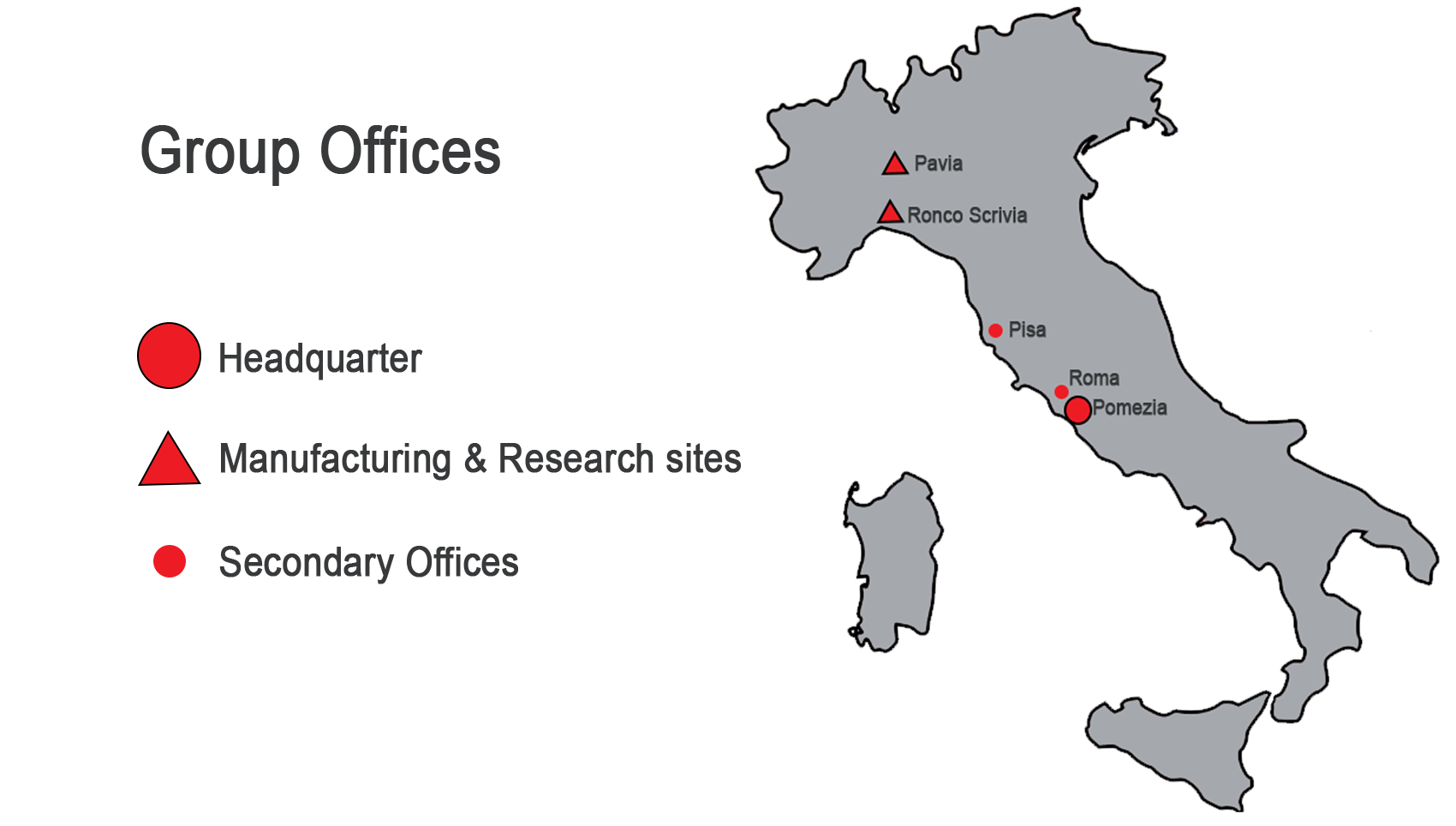
STRUCTURE OF F&S HOLDING
Savio Pharma Italia is an Italian pharmaceutical company created to provide the medical class with innovative instruments for people’s health (medicinal products, medical devices and food supplements). Savio Pharma Italia operates through a field force of medical representatives focused on general and specialist medicine in the main areas of musculoskeletal pathologies, diabetes care and endocrinology, for its own and under license products.
MISSION and VALUES
The mission of Savio Pharma Italia is to ensure a longer, healthier and happier life to people by offering safe and effective products. We pursue this objective with motivation, enthusiasm and a strong sense of responsibility towards the real needs of our partners, either patients, healthcare professionals, distributors or institutions.

• ITALIAN IDENTITY
Among our main values, the most characterising is our totally ITALIAN IDENTITY: the sense of belonging to Italian culture, civilisation and language and more than this the awareness of such belonging. That is why our products are mainly developed, manufactured and/or packaged in Italy.
• RESPONSIBILITY OF OUR ACTION
Savio Pharma Italia, in order to ensure the high quality standards of the services provided to healthcare operators, commits itself to obtain every year the Certification of its procedures concerning the scientific information activity, in compliance with the specific guidelines of Farmindustria, which the company is a member of. Such certification is released by ACCREDIA (the Italian certification Body) after verifying the compliance with reference regulations, especially with Legislative Decree n.219/2016 (which transposes into local law the Community Code concerning medicinal products for human use) and Ministry of Health Decree dated April 30th 2015 concerning pharmacovigilance.
• PASSION AND COMMITMENT FOR OUR WORK
We work to satisfy many different needs, not just material but also psychological and emotional, and the more we are able to meet needs with our work, the more we feel motivated to do it better and better. Always giving our best, both as individuals and as a team.
• AWARENESS OF OUR POTENTIALS AND LIMITS
We work to increase our collaborators and our own competences in order to enhance everyone’s strengths.
• CONSTANT CHECK OF OUR ACTIONS
We constantly pay attention to the immediate effects of our actions in order to promptly make the needed changes to achieve our goals.
• CONTINUOUS AND CIRCULAR, OPEN, CLEAR AND EFFECTIVE COMMUNICATION
We aim at implementing easy and immediate communication processes, allowing everyone to send a comment, express a doubt, make a question, share an observation, exchange news and information.
• POSITIVITY
We strongly believe in positivity, the mental attitude of expecting positive results. A positive mind expects happiness, health and the best possible outcome in every situation. A person who applies a positive approach to life shall always obtain more success in both professional and personal life.
The pursuit of the Company’s interest can never justify a behaviour contrary to the principles of honesty, fairness, legitimacy and transparency. For this reason, the Company’s Administrative Body has adopted the Code of Conduct of F&S Holding S.r.l. which, together with indicating the ethical reference standards, also sets out the rights, duties and responsibilities of all those who, in whichever position, operate within the Company, either as employees, consultants or anyway through a cooperation relationship. The Code of Conduct plays an essential role in enforcing such principles: Savio Pharma Italia S.r.l. commits itself to divulge its contents to everybody it enters into a business relationship with and requests the respect of such Code by all its employees, consultants and/or collaborators. The Code of Conduct also represents a governance instrument and is an integral part of the Company’s “Model of Organisation, Management and Control” for the protection from incriminations according to Legislative Decree n. 231/2001 with reference to the Company’s administrative responsibilities.
ETHICS and TRANSPARENCY
EFPIA Code (Code about the Transparency of Value Transfers among Pharmaceutical Companies, Healthcare Professionals and Healthcare Organisations)

Based on the ethical principles driving its actions and in order to satisfy the more and more pressing and continuous expectations of legality and transparency expressed by both Citizens and Institutions, SAVIO Pharma Italia has adhered with conviction to the EPFIA Code (European Federation of Pharmaceutical Industries and Associations), whose contents have been included in the Code of Conduct of Farmindustria. The correct and positive relationship established between Pharmaceutical Industry and Healthcare Professionals (HCP: Healthcare Professionals) and/or Healthcare Organisations (HCO: Heathcare Organisations) has always lead to research and develop innovative therapies, aimed at safeguarding one of the fundamental and constitutionally guaranteed Citizens’ rights, i.e. the right to health. In fact, it is useful to highlight that the Transfers of Value between the pharmaceutical Industry and the medical Community, which the EFPIA Code refers to, cannot and should not be exclusively considered as transfers of economic nature; they also convey the mutual transfer of a much important value, i.e. the scientific knowledge, for the benefit of Citizens/Patients.
The cooperation between pharmaceutical Industry, healthcare Operators and healthcare Organisations, already well regulated by the current legislation, becomes even more transparent thanks to the application of the EFPIA Code.Such further transparency in relationships shall consolidate the basis for the future collaborations and lead to further benefits for Citizens/Patients that, having the opportunity to know the contents of such relationships, shall be in the condition to:
• receive the proposed treatments with greater trust and awareness;
• overcome the prejudices and concerns, always present in the public Opinion when a reference to economic relationships (Transfers of values) is present within the Health system.
SAVIO Pharma Italia therefore commits itself to yearly publish on its company website all the direct or indirect economic transfers (Transfers of value), both in cash or kind, made to Healthcare Operators and/or healthcare Organisations the year before. The Transfers of Value, which data publication is requested for, are those relative to the participation to Events (Conventions, Congresses, Visits to production plants…), consulting and professional services, donations and contributions, research and development (clinical, non-clinical and observational trials).
For year 2020, please see the relative TABLE
For year 2021, please see the relative TABLE
For year 2022, please see the relative TABLE
The data publication is made on an individual basis. In case an Operator denies the formal authorisation to data publication, according to the current regulations on privacy SAVIO Pharma Italia S.r.l. publishes the data in an aggregate form. All the Transfers of Value are expressed in Euros (€). The payments made for services, sponsorships, contributions, donations and other services in general, are recorded with reference to the year in question (calendar year) and are usually expressed net of VAT.
In case a withholding tax is applied, the same is included. Other typologies of payments such as those made for services related to Travel and Accommodation (travel documents, hotels and so on) should be intended including VAT to the extent permitted by law.
Code of Conduct of Farmindustria
Savio Pharma Italia deems it essential to have ethical, loyal and transparent relationships with all its interlocutors, either healthcare Operators, Institutions, Citizens, Collaborators or commercial Partners. Based on this, the Company operates in the respect of applicable laws and regulations, of professional ethics and of internal regulations; it is a member of Farmindustria and fully complies with the contents of the CODE OF CONDUCT of this association.
|
RELATIONSHIPS WITH PATIENT ORGANISATIONS |
|
In compliance with the rules of the Code of Ethics adopted by Farmindustria (the trade association of Italian pharmaceutical companies), SAVIO Pharma Italia publishes the list of Patient Organisations supported last year, including details on the purposes of such support and the level of funding provided. The year 2022 Report can be found HERE. |
WHISTEBLOWING
“Whistleblowing” report for Savio Group Companies (Farmaci & Salute Holding S.r.l. – I.B.N. Savio S.r.l. – Savio Industrial S.r.l. – Farmaceutici Caber S.r.l. – Savio Pharma Italia S.r.l.)
The Savio Group, in compliance and in pursuant to the provisions referred to in Legislative Decree no. 24 of March 10, 2023, implementing Directive (EU) 2019/1937 of the European Parliament and of the Council of October 23, 2019, on the protection of persons reporting breaches of Union law and national legislation, has implemented a “Whistleblowing” Reporting System containing detailed indications of the subject matter of the reports, as well as the related channels and operational methods for making reports concerning violations of national legislation and EU legislation that harm the public interest or the integrity of the Savio Group.
The key points are outlined in the Information and operational guidelines on whistleblowing by the Savio Group. The processing of the reporter’s data is specified in the Privacy Policy with the corresponding Consent for processing personal data of the reporter to be sent completed to the Reporting Officer “Whistleblowing". The “Whistleblowing” reporting management system involves the use of an IT platform managed with full respect for the protection of the reporter’s identity.
The platform allows for reporting, even anonymously, violations, alleged or known, of rules, laws, procedures, company policies, as well as unlawful behaviors, ensuring the maximum confidentiality of the reporter.
To make a report through the online platform “EQS Integrity Line Professional", the link to be used is https://wbgrupposavio.integrityline.com/.
Our Team
HEADQUARTERS STAFF

MARIA ALLEGRANTI
Commercial Assistent

MANUELA BARTOLINI
Scientific & Training Service

MICHELE BUGLI
Training Manager

SERGIO FAUGNO
Sales & Marketing Director

MARIANNA PANUNZIO
Marketing Assistant
SALES MANAGERS

MASSIMO DE RUBERTO
Responsabile Vendite
Centro - Sud
massimo.deruberto@saviopharma.eu

MARCO PELLIZZONI
Responsabile Vendite
Centro-Nord
marco.pellizzoni@saviopharma.eu
AREA MANAGERS

PIETRO ADAMI
Responsabile di Area
Veneto - Trentino A.A. - Friuli V.G. - Est Lombardia
pietro.adami@saviopharma.eu

MAURIZIO BALSANO
Responsabile di Area
Sicilia - Sud Calabria
maurizio.balsano@saviopharma.eu

ROBERTO MARNATI
Responsabile di Area
Lombardia
roberto.marnati@saviopharma.eu

MATTIA PERATA
Responsabile di Area
Piemonte - Valle D'Aosta - Liguria - Sardegna
mattia.perata@saviopharma.eu

ANGELA PIETROPAOLO
Responsabile di Area
Puglia - Abruzzo - Molise
angela.pietropaolo@saviopharma.eu

FABRIZIO SACCONE
Responsabile di Area
Roma sud
mario.santoro@saviopharma.eu
REPS

ROCCO ALESSANO
Informatore scientifico
Bologna
rocco.alessano@saviopharma.eu

VIVIANA ANATELLA
Informatore scientifico
Napoli Centro
viviana.anatella@saviopharma.eu

FABRIZIO ANGIULLI
Informatore scientifico
Bari - Bat
fabrizio.angiulli@saviopharma.eu

SILVIA ARONE
Informatore scientifico
Varese
silvia.arone@saviopharma.eu

MARCO BACCELLI
Informatore scientifico
Lucca
marco.baccelli@saviopharma.eu

STEFANO BALLETTA
Informatore scientifico
Palermo Est
stefano.balletta@saviopharma.eu

GREGORIO BARILA'
Informatore scientifico
Catanzaro - Reggio Calabria - Vibo Valentia
gregorio.barila@saviopharma.eu

EMILIA BASTA
Informatore scientifico
Bologna - Faenza - Forlì
emilia.basta@saviopharma.eu

PRIMO BELLUCCI
Informatore scientifico
Roma Nord
primo.bellucci@saviopharma.eu

GEMMA BIONDO
Informatore scientifico
Trapani - Agrigento Ovest
gemma.biondo@saviopharma.eu

TIZIANA CANGIANO
Informatore scientifico
Caserta - Napoli Nord
tiziana.cangiano@saviopharma.eu

ANGELA CARBONE
Informatore scientifico
Matera - Potenza
angela.carbone@saviopharma.eu

ANDREA CIFERNI
Informatore scientifico
Chieti - Pescara - Teramo
andrea.ciferni@saviopharma.eu

GIUSEPPE CILIBERTI
Informatore scientifico
Roma Ovest
giuseppe.ciliberti@saviopharma.eu

ANGELA CLEMENTE
Informatore scientifico
Taranto
angela.clemente@saviopharma.eu

CRISTINA COMPARIN
Informatore scientifico
Vicenza
cristina.comparin@saviopharma.eu

MASSIMO CONCINA
Informatore scientifico
Novara - Verbania
massimo.concina@saviopharma.eu

LAURA DALL'ARGINE
Informatore scientifico
Parma
laura.dallargine@saviopharma.eu

LUCIA DEANDREA
Informatore scientifico
Bergamo
lucia.deandrea@saviopharma.eu

ANTONELLO DOMINA
Informatore scientifico
Torino
antonello.domina@saviopharma.eu

FEDERICA FABIANI
Informatore scientifico
Reggio Emilia
federica.fabiani@saviopharma.eu

STEFANO GAIARIN
Informatore scientifico
Gorizia - Trieste - Udine
stefano.gaiarin@saviopharma.eu

SIMONE GIAMMATTEI
Informatore scientifico
Pesaro Urbino - Riccione
simone.giammattei@saviopharma.eu

MAURIZIO GIANNINONI
Informatore scientifico
Grosseto - Livorno - Siena
maurizio.gianninoni@saviopharma.eu

TIZIANA GINOPRELLI
Informatore scientifico
Messina
tiziana.ginoprelli@saviopharma.eu

MARIANGELA GIORGIO
Informatore scientifico
Avellino - Benevento
mariangela.giorgio@saviopharma.eu

PAOLO GULINO
Informatore scientifico
Caltanissetta - Catania - Enna - Ragusa
paolo.gulino@saviopharma.eu

ROSA LA FATA
Informatore scientifico
Catania
rosa.lafata@saviopharma.eu

SALVATORE LEPERA
Informatore scientifico
Catanzaro - Cosenza - Crotone
salvatore.lepera@saviopharma.eu

SILVIA LOMBARDI
Informatore scientifico
Pordenone - Treviso - Udine
silvia.lombardi@saviopharma.eu

MANUELA MALCHAR
Informatore scientifico
Nuoro - Oristano - Olbia Tempio - Sassari
manuela.malchar@saviopharma.eu

LAPO MARINAI
Informatore scientifico
Firenze
lapo.marinai@saviopharma.eu

VINCENZO MATERA
Informatore scientifico
Bari Nord - Barletta
vincenzo.matera@saviopharma.eu

GIOVANNI MIRIZZI
Informatore scientifico
Bari Sud
giovanni.mirizzi@saviopharma.eu

MASSIMILIANO MORETTI
Informatore scientifico
Genova
massimiliano.moretti@saviopharma.eu

LARA PAOLUCCI
Informatore scientifico
Roma
lara.paolucci@saviopharma.eu

LUCA PINNA
Informatore scientifico
Cagliari - Carbonia/Iglesias - Ogliastra - Oristano - Medio Campidano
luca.pinna@saviopharma.eu

FRANCESCO PRATICO'
Informatore scientifico
Cosenza - Reggio Calabria
francesco.pratico@saviopharma.eu

MASSIMILIANO PULVIRENTI
Informatore scientifico
Messina - Siracusa
massimiliano.pulvirenti@saviopharma.eu

ARDUINO RECCHIA
Informatore scientifico
Pisa
arduino.recchia@saviopharma.eu

IMMACOLATA SALATIELLO
Informatore scientifico
Napoli Nord
immacolata.salatiello@saviopharma.eu
ALESSANDRA SAVAR
Informatore scientifico
Milano Sud - Ovest
alessandra.savar@saviopharma.eu

MARIKA SCAGLIONE
Informatore scientifico
Palermo -Trapani
marika.scaglione@saviopharma.eu

TIZIANA SIBILLA
Informatore scientifico
Foggia
tiziana.sibilla@saviopharma.eu

ANTONELLO SIRUGO
Informatore scientifico
Campobasso - Chieti - Foggia - Isernia
antonello.sirugo@saviopharma.eu

SANTA SORIANO
Informatore scientifico
Caserta
santa.soriano@saviopharma.eu

GIORGIO TEDESCHI
Informatore scientifico
Massa - La Spezia
giorgio.tedeschi@saviopharma.eu

LORELLA TERRACCIANO
Informatore scientifico
Napoli Est
lorella.terracciano@saviopharma.eu

FEDERICO TOMASSI
Informatore scientifico
Bologna Ovest - Modena Nord-Est
federico.tomassi@saviopharma.eu

FAUSTA TRIFOGLIO
Informatore scientifico
Alessandria - Asti
fausta.trifoglio@saviopharma.eu

LUDOVICO VASTOLA
Informatore scientifico
Salerno - Napoli Sud-Est
ludovico.vastola@saviopharma.eu

FRANCESCA VENTURI
Informatore scientifico
Prato - Pistoia
francesca.venturi@saviopharma.eu

AGOSTINO VERDOLIVA
Informatore scientifico
Napoli Sud - Salerno
agostino.verdoliva@saviopharma.eu

RINALDO VIGLIONE
Informatore scientifico
Asti - Cuneo
rinaldo.viglione@saviopharma.eu
CERTIFICATIONS
 |
Savio Pharma Italia is certified by CERTIQUALITY for compliance of the Scientific Information activities with the Farmindustria’s Code of Conduct, which prescribes the respect of specific behavioral rules regulating the transparency and ethics of the different company activities, in addition to the compliance with all the applicable laws. Certificate can be downloaded by clicking here. |


